electron configuration for tin|Tin Electron Configuration (Sn) with Orbital Diagram : Clark To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the number of electrons for the . support magento version 2.1 , 2.2 and higher; Create horizontal menu for your magento 2 store; Custom category label; When Customers hover over a Menu item, a list of your products will be displayed. Mega menu extension helps to display categories and corresponding sub-categories; Easily add, delete and edit menu items; responsive with .
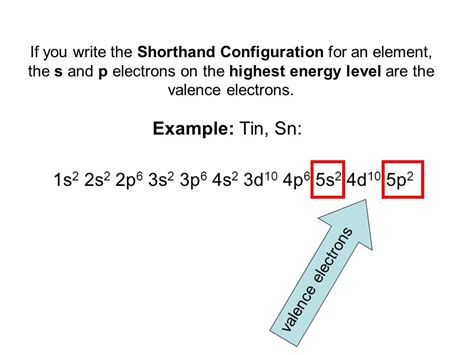
electron configuration for tin,Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds .
electron configuration for tin To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the number of electrons for the . Aufbau principle. First, find electrons of tin atom. Periodic table | Image: Learnool. The atomic number of tin represents the total number of electrons of tin. Since the atomic number of tin is 50, the total electrons of tin .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point . Tin Electron Configuration. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4.
Tin electron configuration. ← Electronic configurations of elements. Sn (Tin) is an element with position number 50 in the periodic table. Located in the V period. Melting point: 232 ℃. .Tin is a post-transition metal with atomic number 50 and chemical symbol Sn. Its electron configuration is [Kr] 4d 10 5s 2 5p 2, with valence electrons 4 and valency electrons 2,4.Electrons & Oxidation. Nuclear. Isotopes. Mass Number. The sum of the number of protons and neutrons of an atomic nucleus. In other words, it's the sum of the number of nucleons in an .Tin. Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. indium ← tin → antimony. Tin, complete electron configuration.
Tin, complete electron configuration. © 2009-2016 | www.prvky.com | kontaktkontakt
electron configuration for tin Tin Electron Configuration (Sn) with Orbital DiagramFor example, the atomic number of Sodium is 11 and its electron configuration is 1 s 2 2 s 2 2 p 6 3 s 1; Electronic configuration of Tin:-Atomic number of Tin is 50, that is Tin has a total of 50 electrons. Since 1s orbital can hold a maximum of 2 electrons, so first 2 electrons will fill the 1s orbital, and the next 2 electrons will fill the .Electronic configuration of the Tin atom. Valence electrons. Orbital diagram. Tin electron configuration. ← Electronic configurations of elements . Sn (Tin) is an element with position number 50 in the periodic table. Located in the V period. Melting point: 232 ℃.
Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure.The chemical symbol for Tin is Sn. Electron Configuration and Oxidation States of Tin. Electron configuration of Tin is [Kr] 4d10 5s2 5p2. Possible oxidation states are +2,4. Electron Configuration. The periodic table is a tabular .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the .

This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait — you can avoid all this if you use our .

And thus 50 electrons must be distributed according to the usual #"aufbau"# scheme...Tin lies in Group 14, and thus should have a similar electronic configuration to carbon. Can you demonstrate the similarity.
Tin Electron Configuration (Sn) with Orbital Diagram And thus 50 electrons must be distributed according to the usual #"aufbau"# scheme...Tin lies in Group 14, and thus should have a similar electronic configuration to carbon. Can you demonstrate the similarity. Tin - Sn, on the periodic table is found in the fourteenth column of the periodic table Group IVB this is the second column of the p block. Tin is in the fifth energy level (row). This means that Tin must end with an electron configuration of 5p^2 The total electron configuration would be 1s^2 2s^2 2P^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^2 The .
To find the number of valence electrons for Tin (Sn) we need to look at its electron configuration. This is necessary because Sn is a transition metal (d bl.
Tin is a chemical element of the periodic table with chemical symbol Sn and atomic number 50 with an atomic weight of 118.711 u and is classed as a post-transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 2: 5p 2: Electrons per shell: 2, 8, 18, 18, 4: Valence electrons : 4: Valency .Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at .
Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional figure below, the first and most inner electron shell is represented by blue electrons, the second electron .Protons and Neutrons in Tin. Tin is a chemical element with atomic number 50 which means there are 50 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol .
Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 2; Electrons per Energy Level: . Tin - Sn (EnvironmentalChemistry.com)- Comprehensive information for the element Tin - Sn is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to .Abbreviated electronic configuration of Tin. The ground state abbreviated electronic configuration of Neutral Tin atom is [Kr] 4d10 5s2 5p2. The portion of Tin configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Kr]. For atoms with many electrons, this notation can become lengthy and so an abbreviated .
The tin orbital diagram is a graphical representation of the electron configuration of the tin atom. This diagram shows how the electrons in the tin atom are arranged in different orbitals.Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 .A. Use the periodic table to determine the electron configuration for tin. Express your answer in condensed form, in the order of orbital filling. For example, [He]2s22p2 would be entered as [He]2s^22p^2. B. Enter an electron configuration for iodine based on its .
electron configuration for tin|Tin Electron Configuration (Sn) with Orbital Diagram
PH0 · Tin, electron configuration
PH1 · Tin electron configuration
PH2 · Tin Electron Configuration (Sn) with Orbital Diagram
PH3 · Tin (Sn)
PH4 · Tin
PH5 · Electron configuration for Tin (element 50). Orbital diagram
PH6 · Electron Configuration for Sn, Sn 2+, and Sn 4+
PH7 · Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+)
PH8 · Chemistry of Tin (Z=50)